Almirall, a global pharmaceutical company focused exclusively on medical dermatology, has announced plans to initiate a Phase III clinical study evaluating the efficacy and safety of lebrikizumab in adult patients with nummular eczema. The study, named LumiNE, is scheduled to begin in the first half of 2026 and represents a strategic milestone in Almirall’s ongoing commitment to address unmet needs in chronic inflammatory skin diseases.
Lebrikizumab (Ebglyss) is currently approved for the treatment of moderate-to-severe atopic dermatitis. Through this new Phase III program, Almirall aims to explore the potential of lebrikizumab in a distinct yet related dermatological condition—nummular eczema—where treatment options remain limited and disease burden is high. The LumiNE study is part of Almirall’s broader lifecycle management strategy to expand the therapeutic reach of its advanced biologic medicines and bring innovative solutions to patients with dermatological diseases characterized by chronic inflammation and impaired quality of life.
Although nummular eczema is clinically distinct from atopic dermatitis and requires a tailored treatment approach, emerging scientific evidence suggests overlapping disease mechanisms. In particular, the cytokine interleukin-13 (IL-13) has been identified as a key mediator in both conditions. IL-13 plays a central role in driving inflammation, pruritus, eosinophilic infiltration, and skin barrier dysfunction—hallmark features of nummular eczema. Given lebrikizumab’s high affinity and selective binding to IL-13, targeting this pathway represents a promising therapeutic strategy for patients who do not respond adequately to standard topical treatments.
Commenting on the announcement, Karl Ziegelbauer, Chief Scientific Officer at Almirall, highlighted the potential clinical impact of the study. He stated that the company is excited to advance lebrikizumab into Phase III development for nummular eczema, noting that the biologic’s established efficacy in atopic dermatitis, combined with growing evidence supporting the role of IL-13 in nummular eczema, could enable meaningful progress for patients living with this debilitating disease. According to Ziegelbauer, the LumiNE study aligns with Almirall’s purpose of transforming patients’ lives by expanding access to targeted, science-driven dermatological therapies.
The clinical importance of the study was also emphasized by the LumiNE coordinating investigator, Kilian Eyerich, Medical Director of the Department of Dermatology and Venereology at Medical Center University of Freiburg. Professor Eyerich noted that nummular eczema is frequently underrecognized and difficult to manage, leaving many patients inadequately controlled with existing therapies. He underscored that recent scientific insights into the role of IL-13 in disease development support the rationale for evaluating lebrikizumab in this indication, and expressed anticipation for the study’s initiation and outcomes.
About the LumiNE Study
LumiNE (M-27501-30) is designed as a Phase III, randomized, double-blind, placebo-controlled, parallel-group, multicenter clinical trial, including a double-blind extension phase. The study will evaluate the efficacy, safety, and tolerability of lebrikizumab over a treatment period of up to 48 weeks in adult patients with nummular eczema who are inadequately controlled with topical corticosteroids or for whom such treatments are not medically advisable.
Approximately 270 participants will be initially randomized across around 60 clinical centers throughout Europe, with the potential to enroll additional patients during an extension phase. The primary endpoint of the study is improvement in the Investigator’s Global Assessment of Nummular Eczema (IGA-NE). Key secondary endpoints include reduction in itch severity measured by the Pruritus Numerical Rating Scale (NRS) and changes in patient quality of life assessed using the Dermatology Life Quality Index (DLQI).
About Nummular Eczema
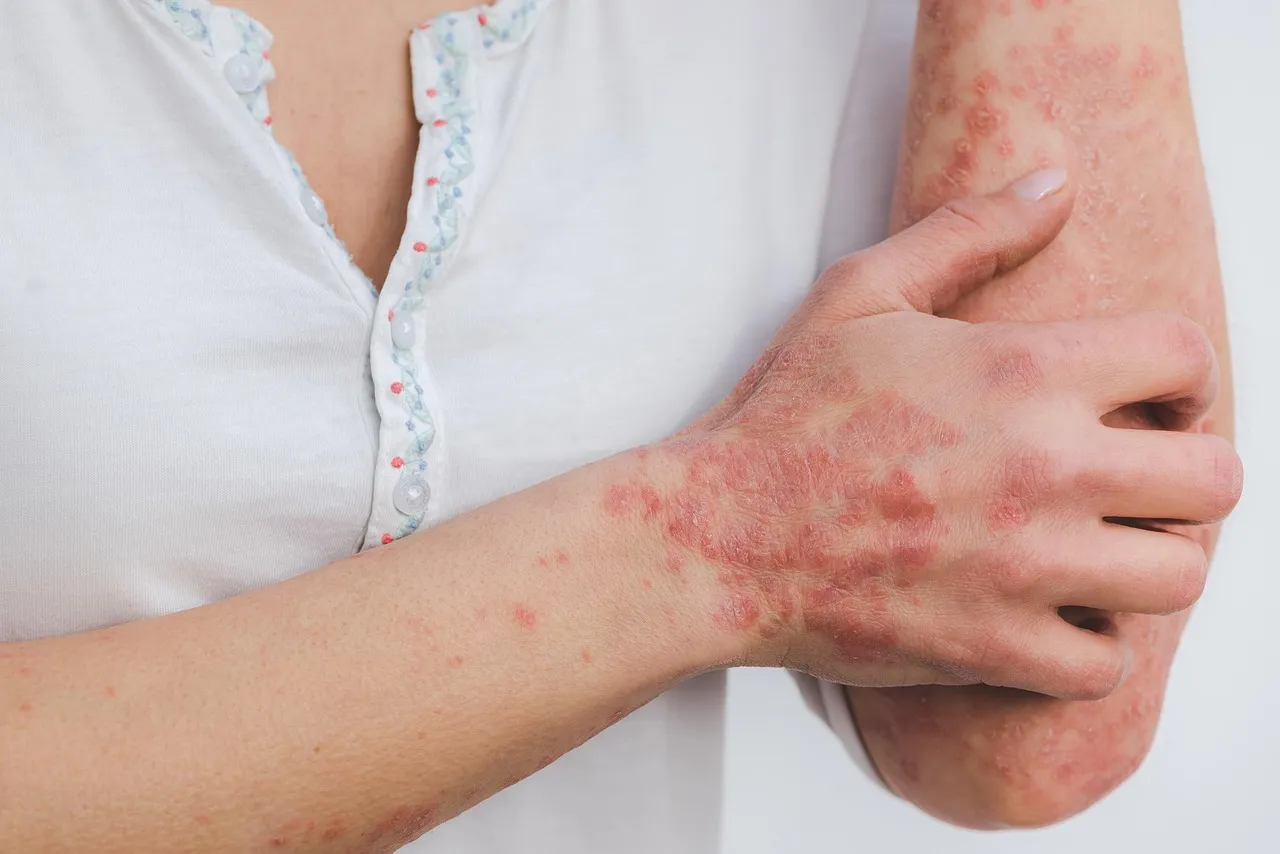
Nummular eczema, also known as discoid eczema, is a chronic, relapsing inflammatory skin disorder characterized by coin-shaped, erythematous, scaly, and intensely pruritic lesions. The condition can occur in individuals with or without atopic dermatitis and is often challenging to diagnose due to its resemblance to other inflammatory dermatoses. Persistent itch, sleep disruption, and visible skin lesions significantly impair patients’ quality of life and contribute to psychosocial burden.
Prevalence estimates for nummular eczema range widely, from approximately 0.1% to 9%, with higher rates observed in older adults and certain ethnic populations. While the disease shares features such as type 2 immune activation and skin barrier dysfunction with atopic dermatitis, it is increasingly recognized as a distinct condition that requires specific diagnostic criteria and targeted therapeutic approaches.
About Lebrikizumab
Lebrikizumab is a monoclonal antibody with high affinity for IL-13, a central cytokine in type 2 inflammatory pathways. By selectively binding IL-13, lebrikizumab prevents formation of the IL-13Rα1/IL-4Rα receptor complex and inhibits downstream signaling involved in inflammation, itch, skin thickening, and barrier disruption. This selective mechanism of action contributes to its favorable efficacy and safety profile.
Ebglyss was approved in Europe in October 2023 and in the United States in August 2024 for the treatment of moderate-to-severe atopic dermatitis. Almirall holds exclusive rights to develop and commercialize Ebglyss for dermatology indications in Europe, while its partner Lilly retains rights in the U.S. and other regions worldwide.
About Almirall
Almirall is a global pharmaceutical company dedicated to advancing medical dermatology through close collaboration with scientists, healthcare professionals, and patients. The company focuses on delivering differentiated, science-based innovations that address unmet needs in dermatological disease, with the ultimate goal of improving patients’ lives and enabling better long-term outcomes.
Source link : https://www.businesswire.com/