hape Memory Medical Inc., developer of the only commercially available volume-expanding shape memory polymer for endovascular embolization, has announced that it has reached 50% enrollment in its ongoing AAA-SHAPE Pivotal Trial. This milestone marks the successful treatment of the 90th patient in the company’s prospective, multicenter, randomized, open-label trial evaluating the safety and effectiveness of the IMPEDE-FX RapidFill® Device to improve abdominal aortic aneurysm (AAA) sac behavior when used in conjunction with endovascular aneurysm repair (EVAR).
The landmark patient procedure was performed by Dr. Aleem Mirza, Principal Investigator and Vascular Surgeon at the Orlando Health Heart and Vascular Institute in Orlando, Florida.
“We congratulate Dr. Mirza and the entire Orlando Health team for enrolling the 90th patient in the AAA-SHAPE Pivotal Trial,” said Ted Ruppel, President and Chief Executive Officer of Shape Memory Medical. “AAA-SHAPE is the first randomized controlled trial to compare standard EVAR to EVAR combined with sac management using IMPEDE-FX RapidFill. The results of this study will be critical in assessing the potential of IMPEDE-FX RapidFill to promote aneurysm sac regression and improve outcomes for patients with AAA.”
Advancing AAA Treatment Through Innovation
AAA-SHAPE, short for Abdominal Aortic Aneurysm Sac Healing and Prevention of Expansion, is designed to enroll 180 patients diagnosed with infrarenal abdominal aortic aneurysms across up to 50 clinical sites in the United States, Europe, and New Zealand.
Participants are being randomized 2:1—either to the treatment arm (EVAR plus sac management with IMPEDE-FX RapidFill) or the control arm (standard EVAR alone). The study’s primary endpoints will measure changes in aneurysm sac diameter and volume, as well as rates of endoleak and secondary interventions required after the procedure.
By studying the behavior of the aneurysm sac after repair, researchers aim to address one of the most persistent challenges in EVAR: failure of the aneurysm sac to shrink. Persistent or expanding sacs after EVAR are linked to higher risks of rupture, reintervention, and mortality.
The IMPEDE-FX RapidFill® Device
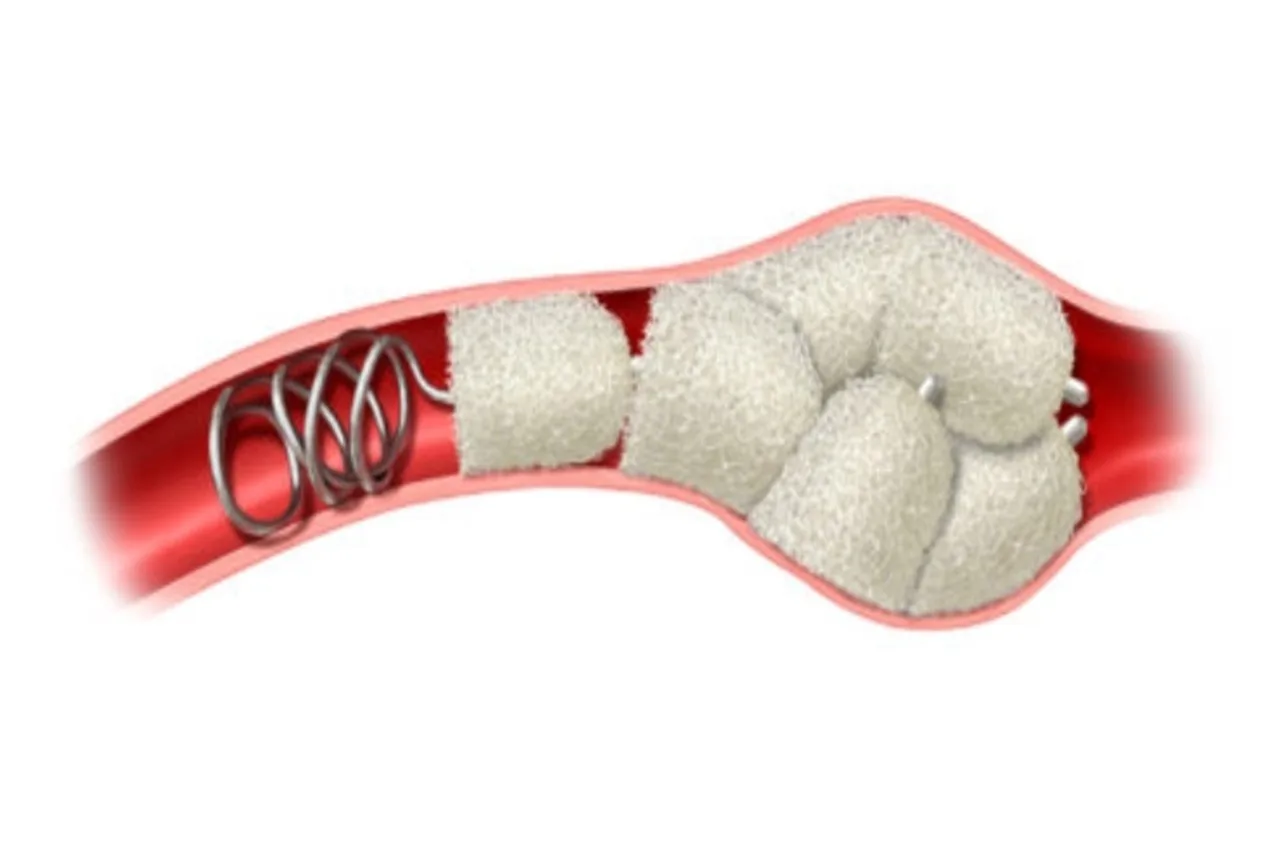
The IMPEDE-FX RapidFill® device utilizes Shape Memory Medical’s proprietary shape memory polymer (SMP) technology—a porous, radiolucent embolic scaffold engineered to expand upon contact with blood. Crimped for catheter delivery, the polymer self-expands in situ, conforming to the aneurysm cavity and promoting stable thrombus formation and sac regression.
Within the AAA-SHAPE trial, IMPEDE-FX RapidFill is deployed into the aneurysm lumen surrounding a commercially available EVAR stent graft. The polymer acts as a volume-filling scaffold, supporting long-term healing by reducing flow, inducing thrombosis, and encouraging fibrotic remodeling of the aneurysm sac.
This innovative approach seeks to address the limitations of conventional EVAR, which primarily focuses on sealing the aneurysm but often fails to promote natural healing or regression of the aneurysm sac.
Building on Early Feasibility Success
Before launching the pivotal study, Shape Memory Medical conducted early feasibility studies in New Zealand and the Netherlands, enrolling 35 patients. Those preliminary results demonstrated encouraging outcomes in sac regression and device safety.
Three-year follow-up data from these feasibility studies are expected to be presented at the upcoming VEITH Symposium in November, offering important insights into the long-term safety and performance of the IMPEDE-FX RapidFill device in clinical use.
“Published data show that approximately 60% of abdominal aortic aneurysms fail to regress—or even expand—within the first year following EVAR,” explained Dr. Virendra Patel, MD, MPH, AAA-SHAPE Global Co-Principal Investigator and Chief of Vascular Surgery and Endovascular Interventions at NewYork-Presbyterian/Columbia University Irving Medical Center. “Lack of regression is strongly correlated with higher rates of reintervention, rehospitalization, and mortality. By promoting sac regression, IMPEDE-FX RapidFill could significantly improve long-term patient outcomes.”
Dr. Patel added that the continued enrollment progress reflects growing enthusiasm across the vascular community:
“We are grateful to all participating centers actively recruiting patients. This study will help define the potential role of sac management as an integral part of future endovascular aneurysm repair strategies.”
Clinical Momentum and Physician Support
The 90th-patient milestone demonstrates the strong momentum behind the AAA-SHAPE trial and growing physician confidence in the potential of shape memory polymer technology.
“We are proud to have treated the 90th patient in the AAA-SHAPE Pivotal Trial,” said Dr. Aleem Mirza of Orlando Health. “The IMPEDE-FX RapidFill’s unique volume-expanding properties may offer meaningful advantages in reducing sac pressurization and improving post-EVAR outcomes. We look forward to seeing the clinical insights this study will bring.”
Shape Memory Medical expects to complete enrollment and present top-line results after sufficient patient follow-up to evaluate changes in aneurysm sac morphology and freedom from adverse events. If successful, the data could support broader use of IMPEDE-FX RapidFill as a complementary therapy for EVAR procedures worldwide.
Transforming Endovascular Aneurysm Repair
Abdominal aortic aneurysms remain a serious and potentially fatal condition, particularly in older adults. While EVAR has become the standard of care for many patients, long-term durability issues—such as sac enlargement and endoleaks—continue to challenge clinicians.
By integrating Shape Memory Medical’s volume-expanding polymer technology, the AAA-SHAPE trial aims to demonstrate a new paradigm in aneurysm management—one that promotes true healing of the aneurysm sac rather than mere exclusion from circulation.
“Our mission is to redefine what’s possible in endovascular repair,” said Ted Ruppel. “With IMPEDE-FX RapidFill, we are exploring a transformative approach that may lead to better sac regression, fewer reinterventions, and ultimately improved survival for AAA patients.”
As Shape Memory Medical progresses toward full trial enrollment, the company continues to work closely with investigators around the world to advance innovation in vascular medicine. The AAA-SHAPE Pivotal Trial stands as a major step toward validating shape memory polymer embolic technology as a cornerstone of next-generation endovascular therapies.